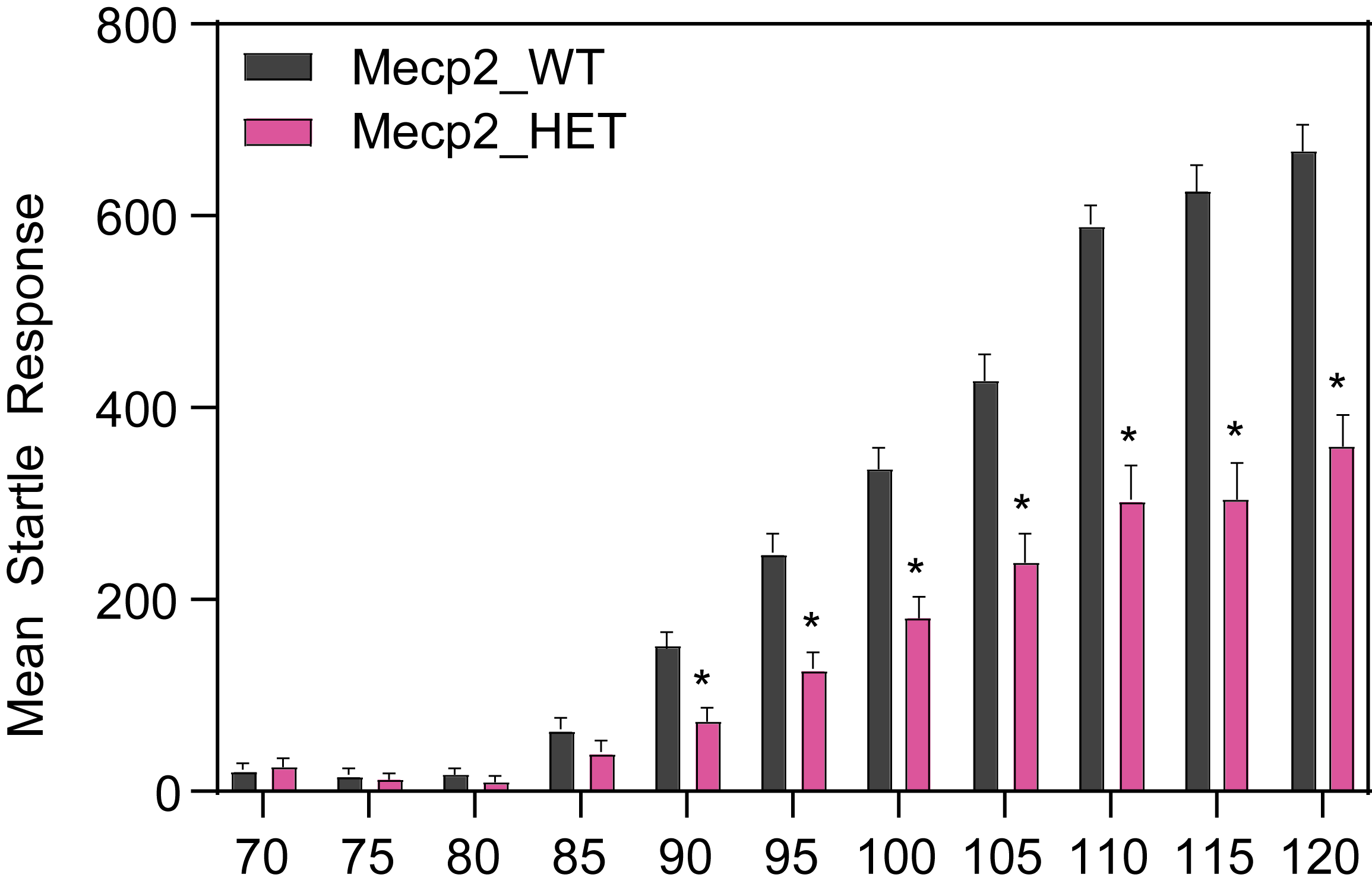
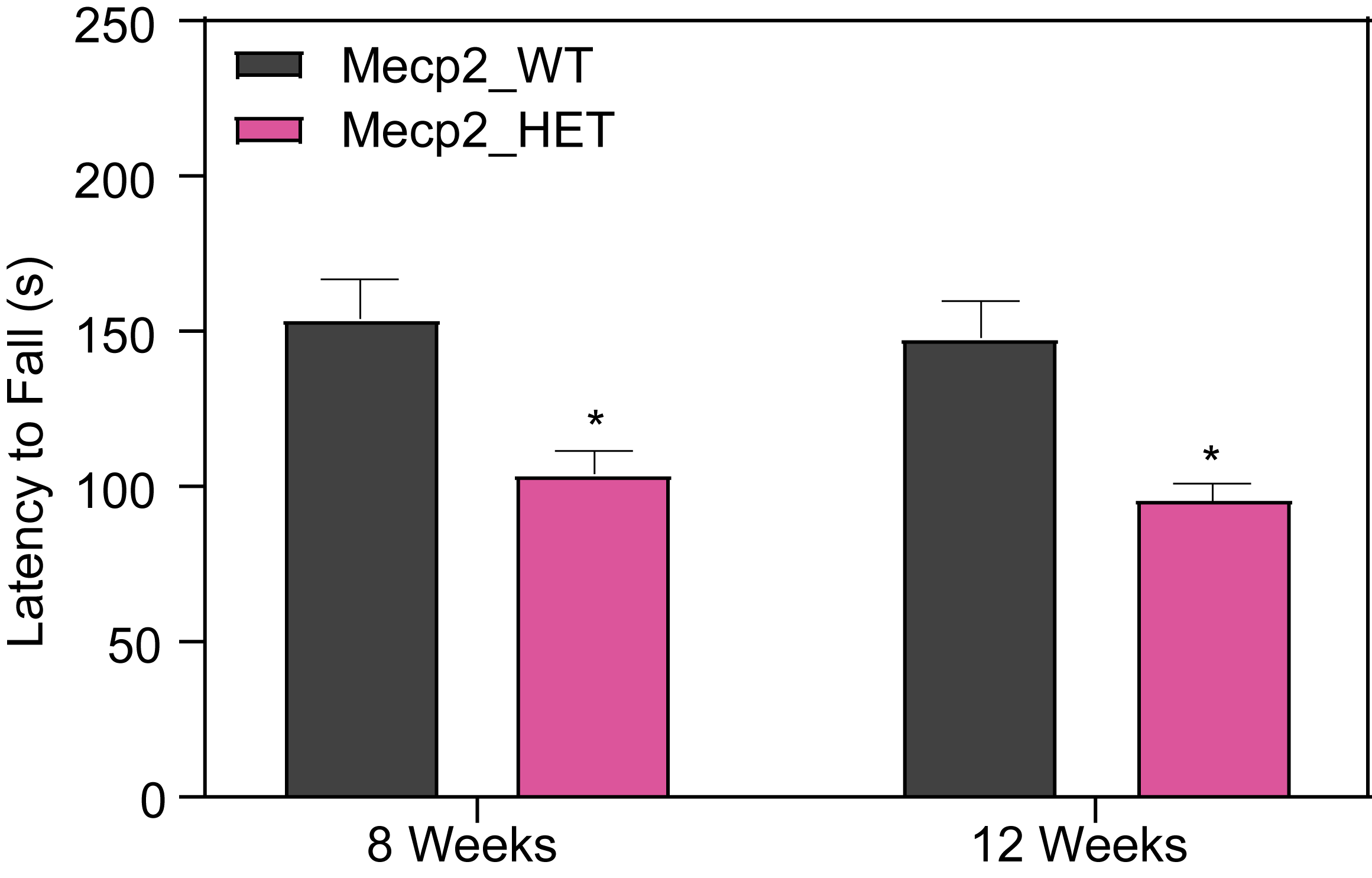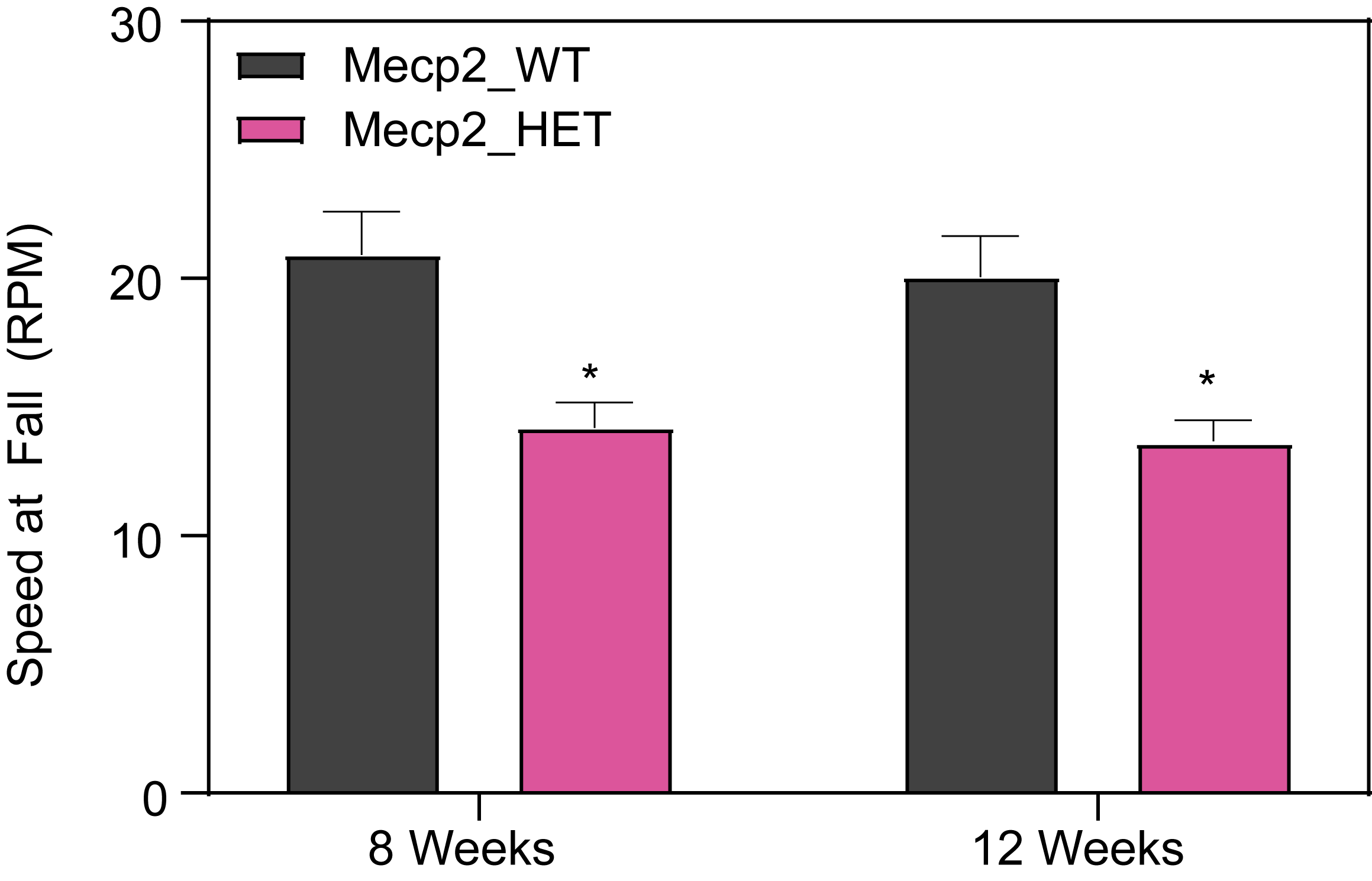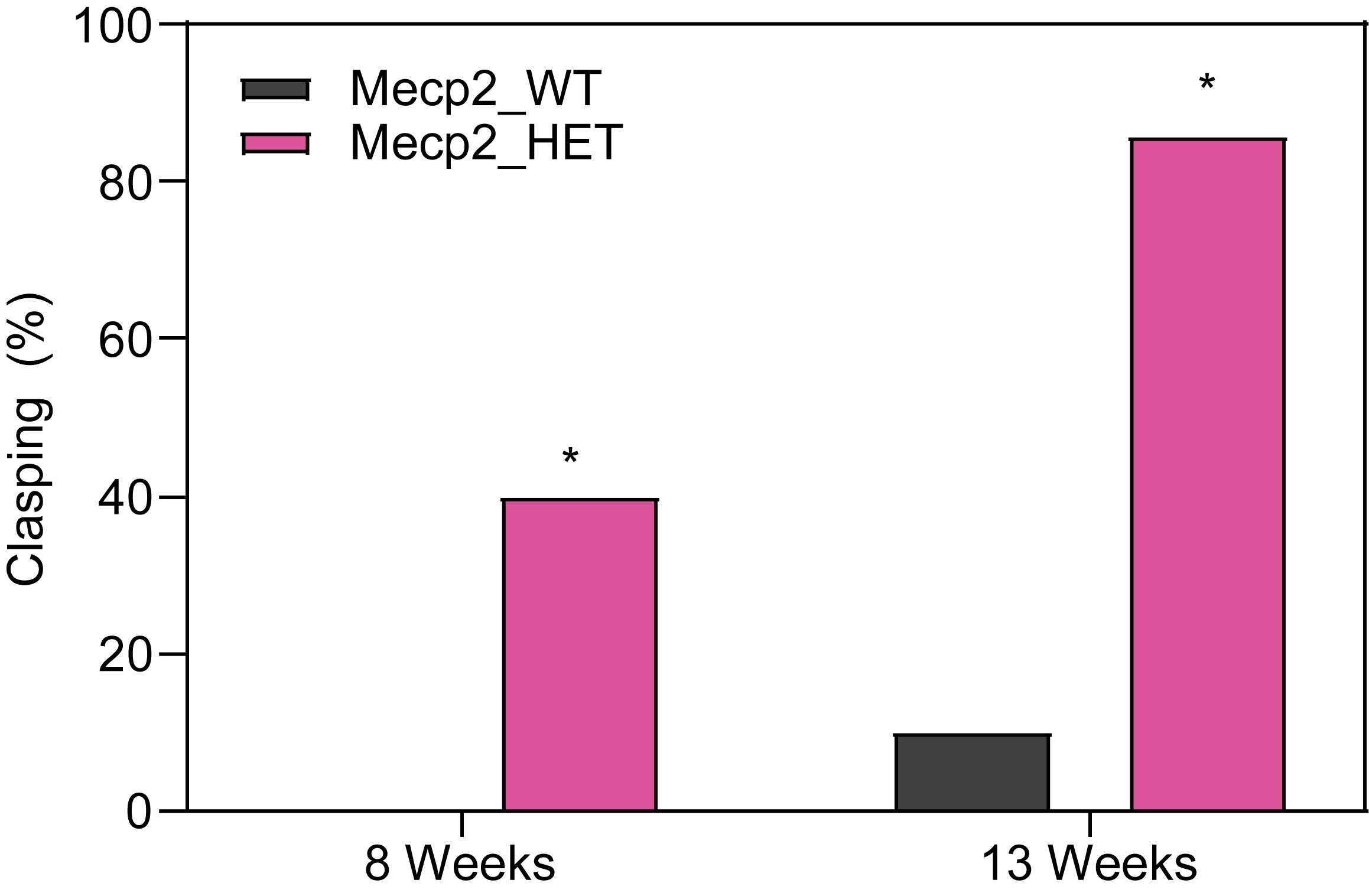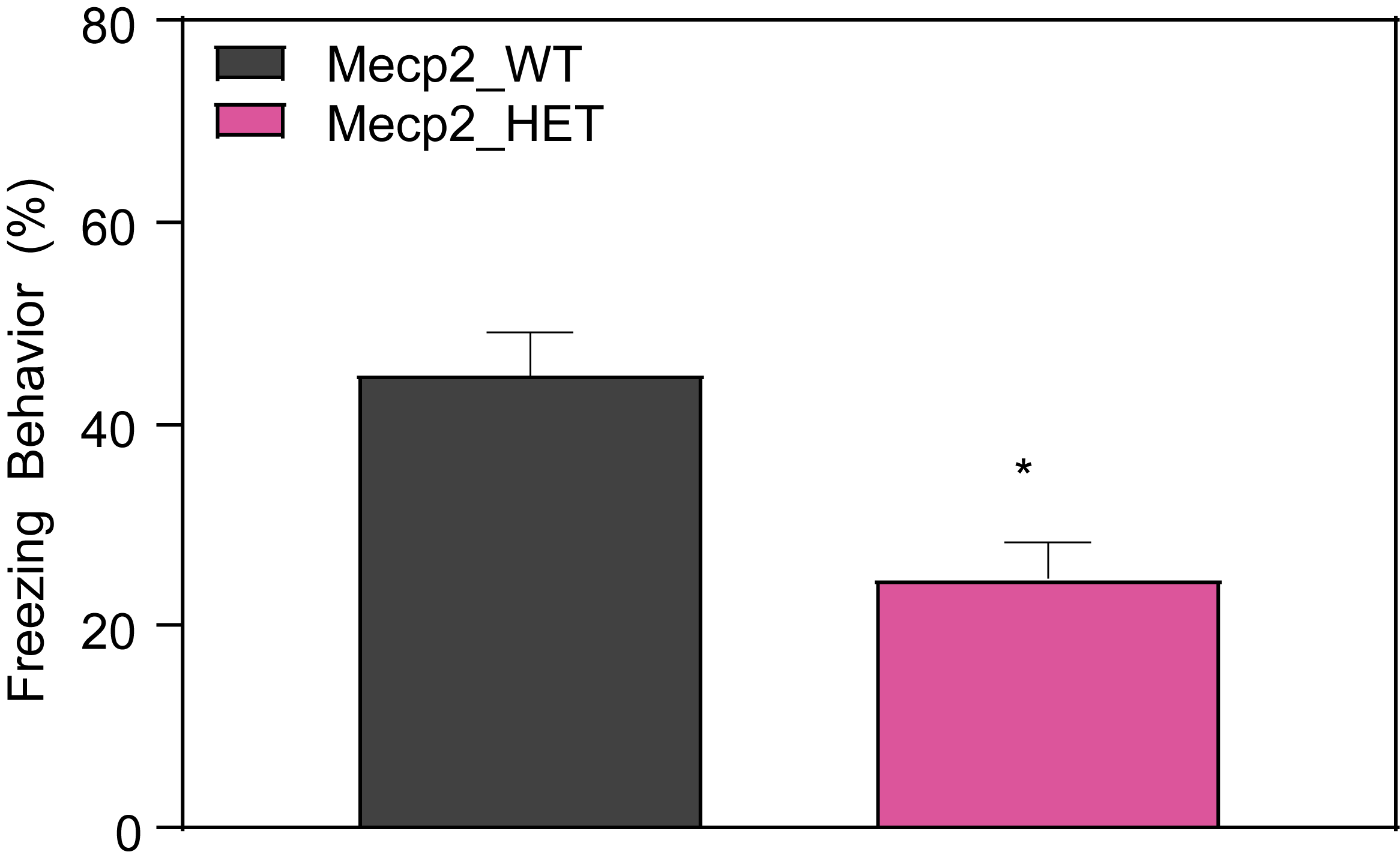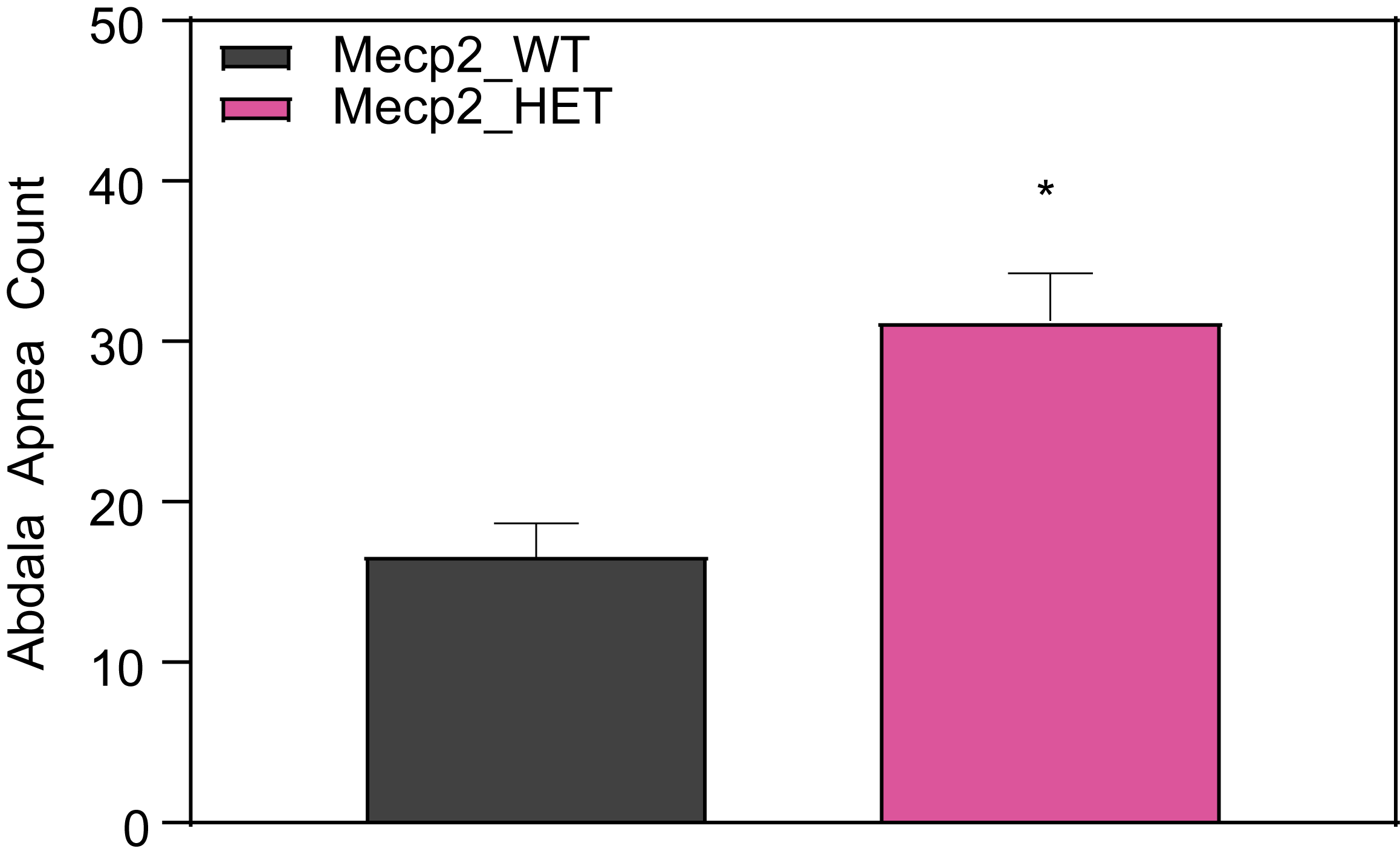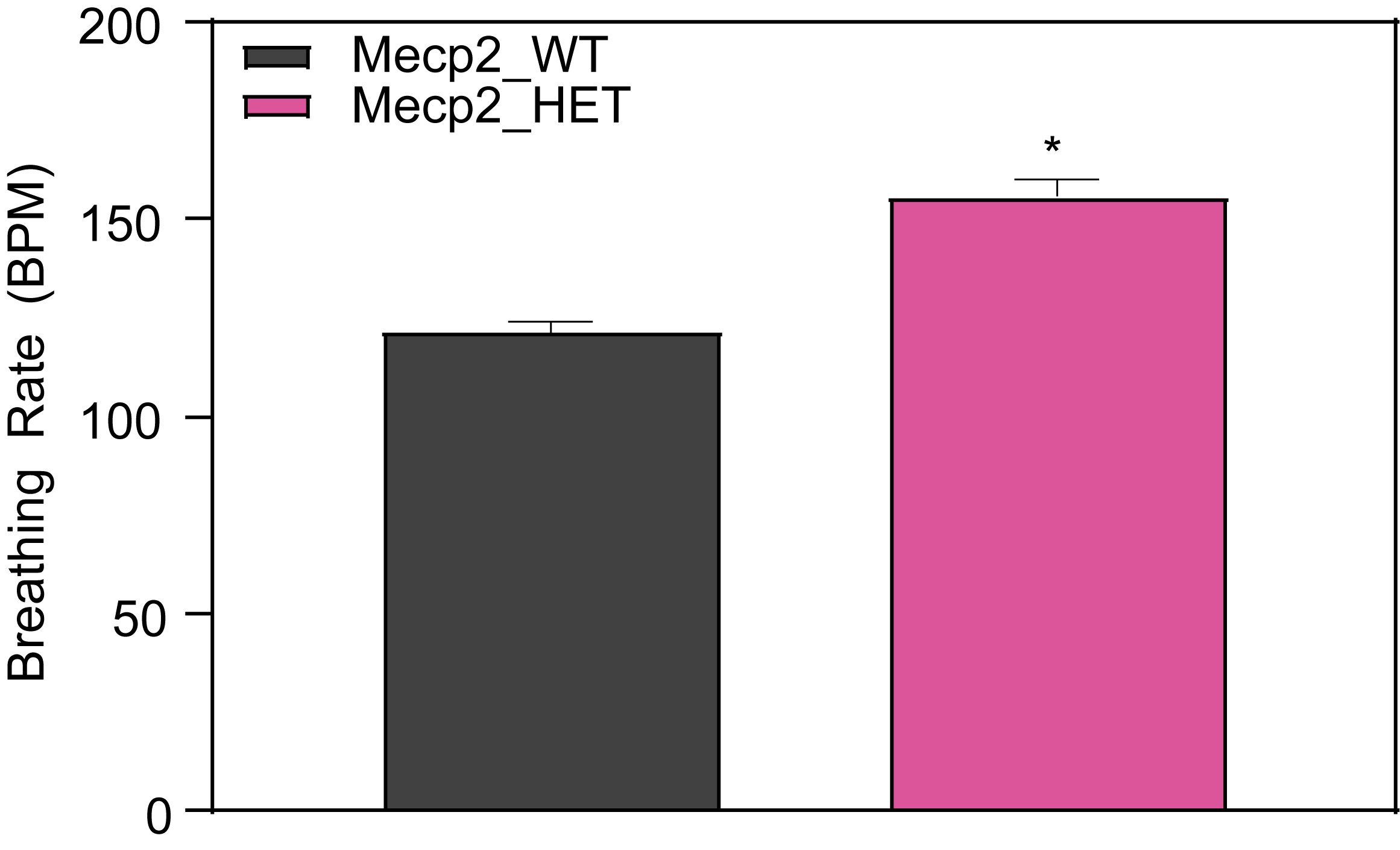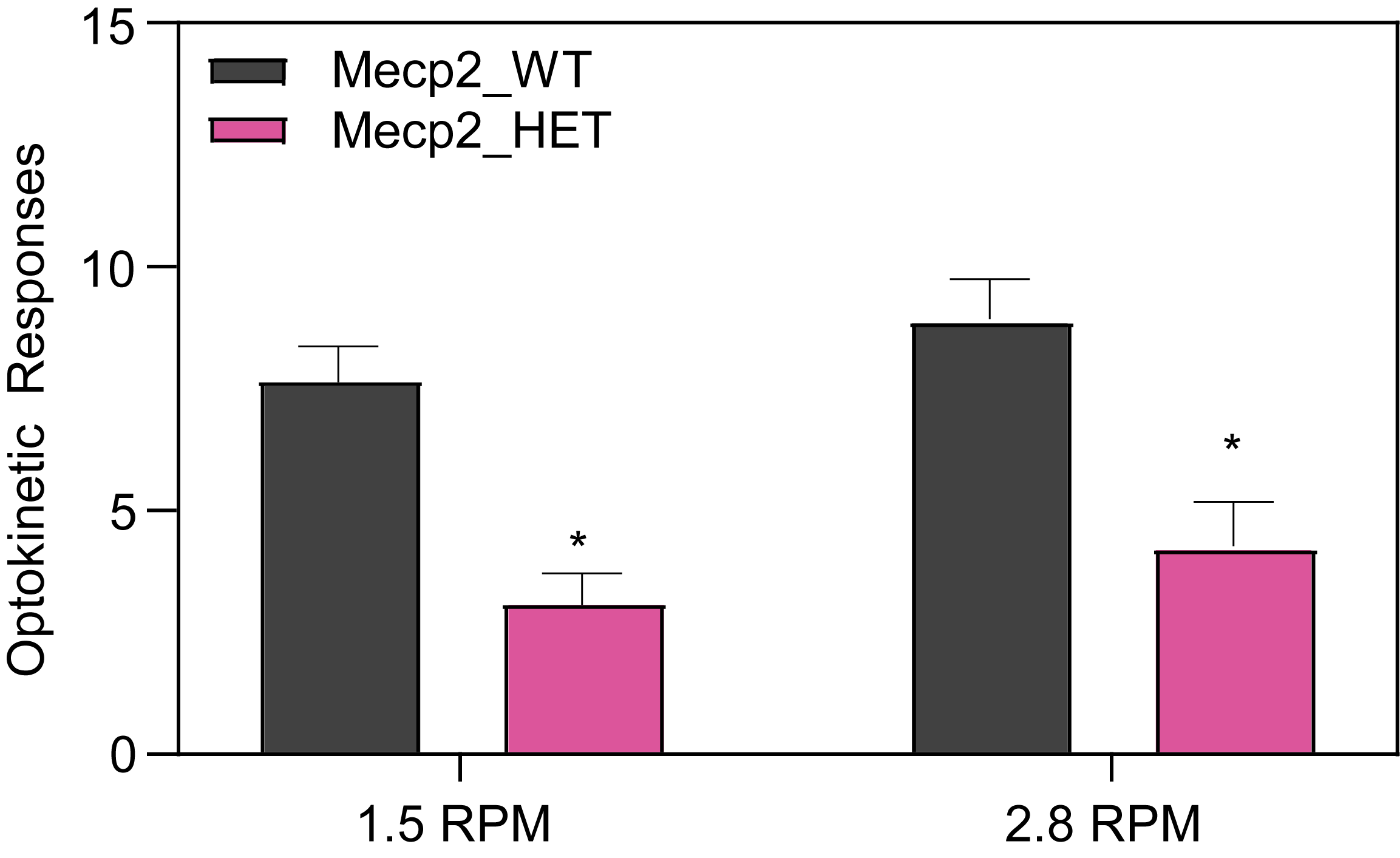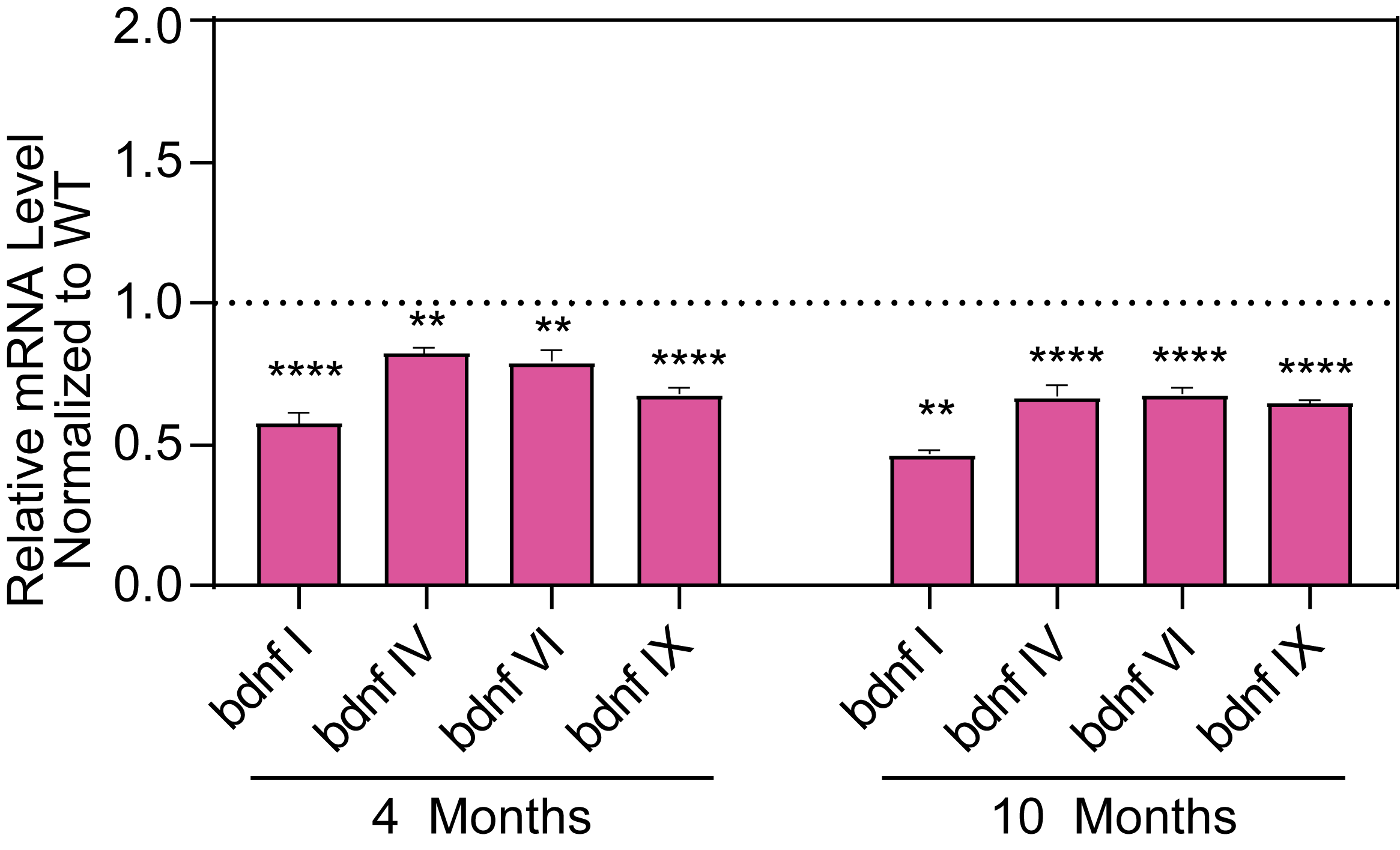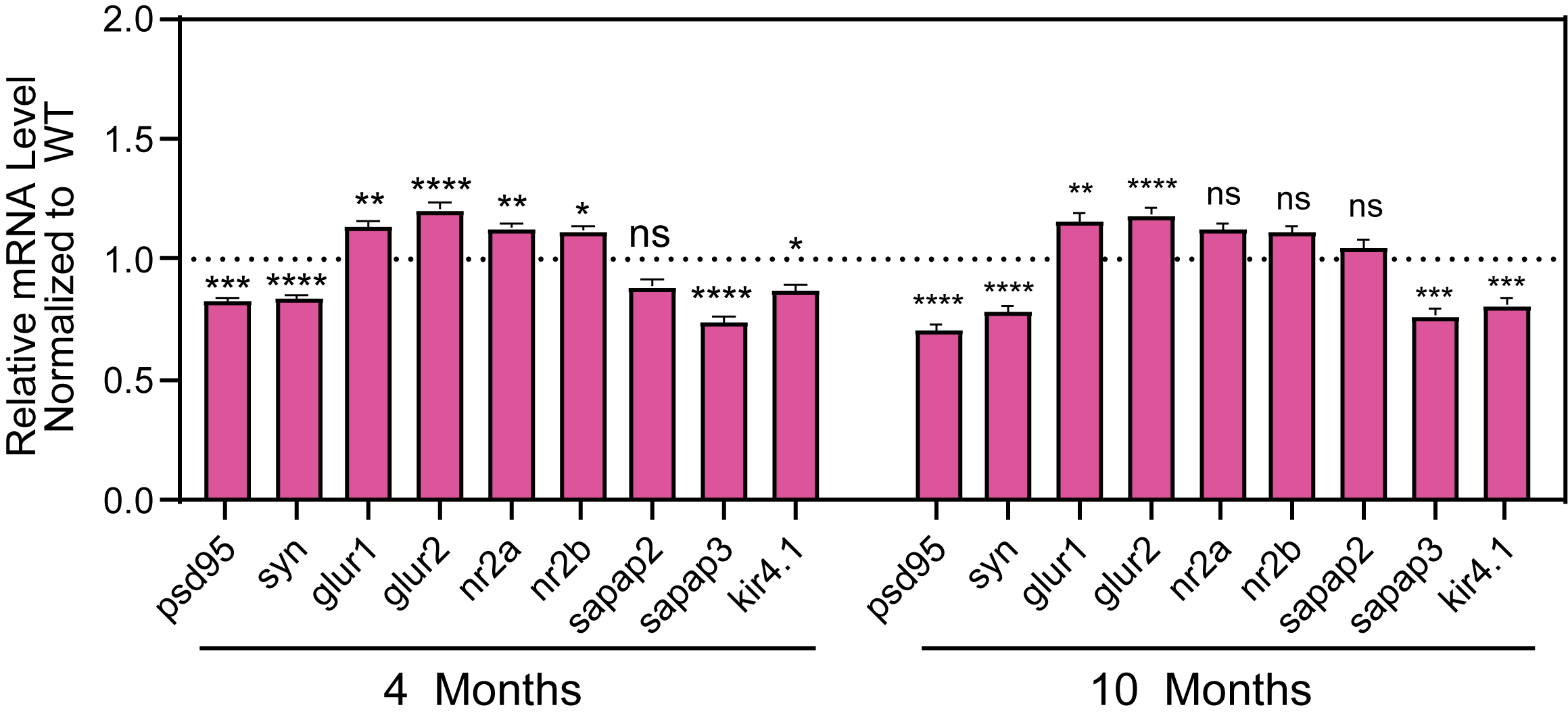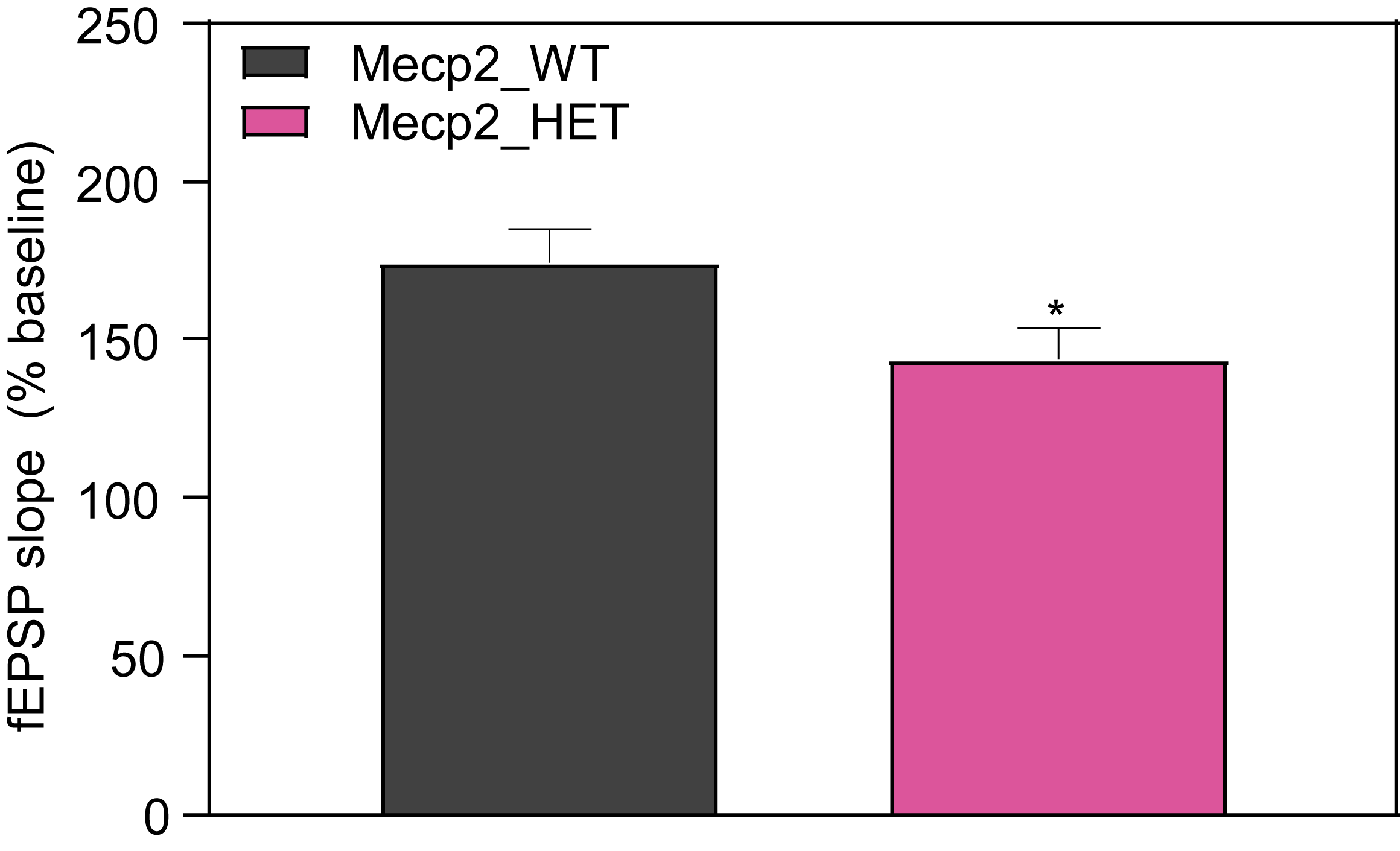Rett syndrome is a neurodevelopmental disorder caused by mutations in the X-linked methyl CpG binding protein 2 (Mecp2) gene. Primarily affecting girls, it stands as a leading cause of intellectual disabilities. Preclinical studies of Rett syndrome are pivotal for advancing treatment development. Our team brings extensive preclinical research experience to support you in discovering the next therapeutic breakthrough for Rett syndrome.
Mecp2 Mouse Model of Rett Syndrome mode of Rett Syndrome
Mice engineered with a knockout allele of the fragile X mental retardation syndrome 1 gene (Fmr1) on the X chromosome serve as a critical model for studying Fragile X syndrome, mirroring many of the phenotypic characteristics observed in humans with the condition, such as hyperactivity, repetitive behavior, and seizures. This genetic modification results in the absence of the Fragile X mental retardation protein (FMRP), a change that triggers the activation of the RAC1 protein. The consequence of this activation is a series of abnormalities in dendritic spines across various brain regions, leading to altered synaptic function. These alterations are pivotal in understanding the neurobiological underpinnings of Fragile X syndrome, as they provide insight into the disorder’s impact on neuronal architecture and connectivity.
The lack of FMRP in these mice not only affects the physical structure of neurons but also significantly alters synaptic plasticity. This alteration manifests as impaired long-term potentiation in the cortex and hippocampus, alongside an increased long-term depression in both the hippocampus and cerebellum. Such changes in synaptic behavior are essential for understanding the learning and memory deficits associated with Fragile X syndrome. Male FMR1 knockout (KO) mice, particularly those bred on an FVB/n background at PsychoGenics, are extensively utilized in research studies. They offer a valuable tool for exploring the mechanisms of Fragile X syndrome and developing potential therapeutic strategies to mitigate its effects.
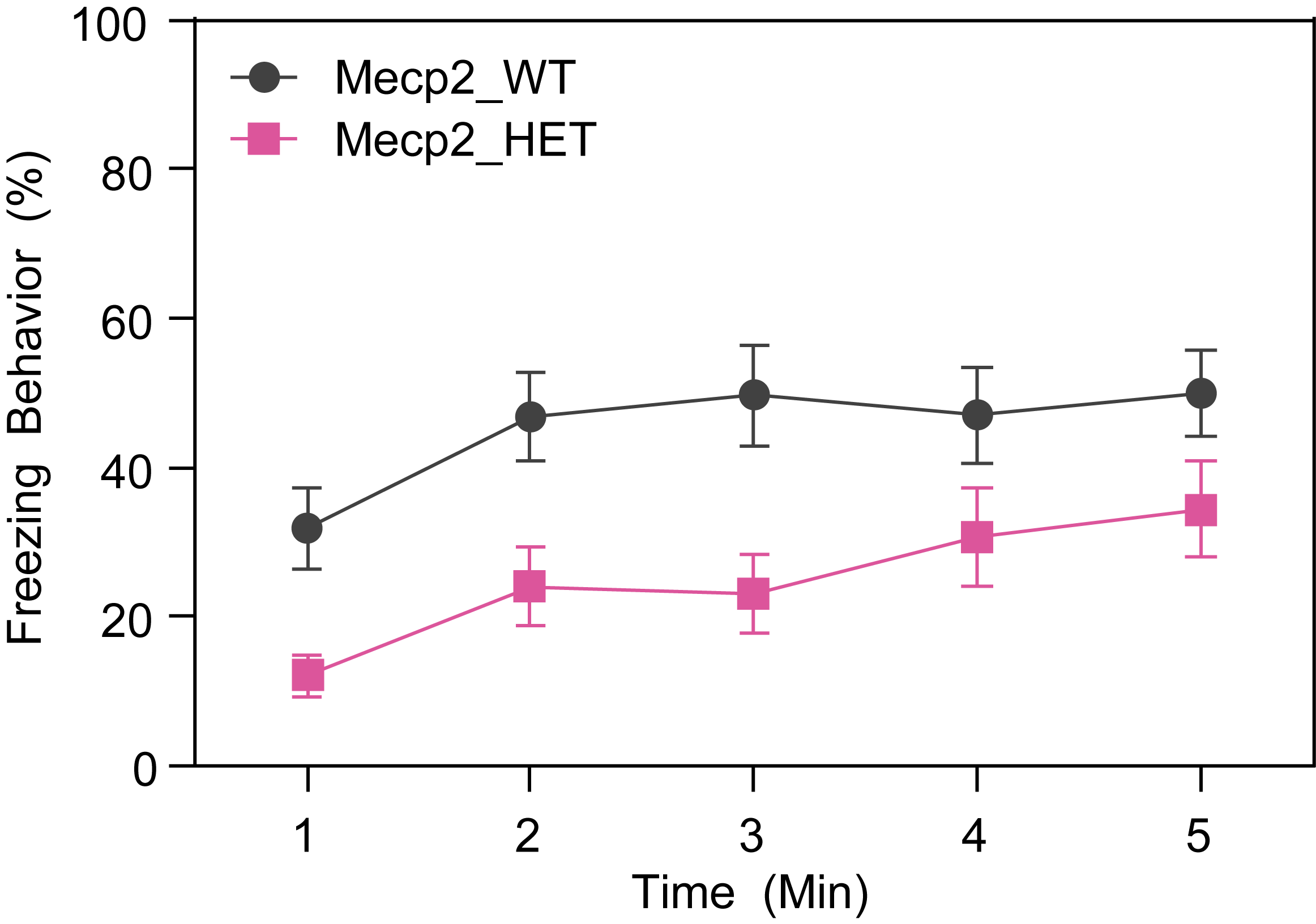
Respiration and Optokinetic Response (6-7 Month Old Mice)
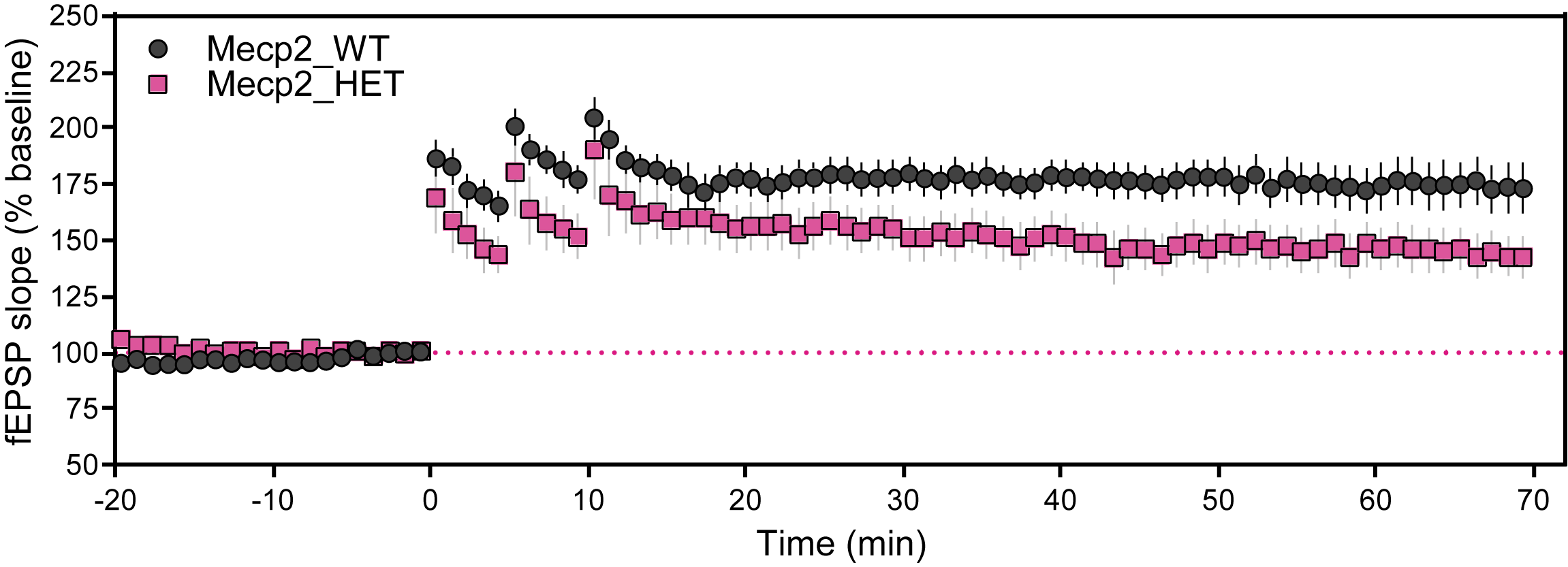
Molecular Biomarkers and Electrophysiology
Driving Your Preclinical Rett Syndrome Research Forward
At PsychoGenics, we collaborate closely with the leading researchers in Rett syndrome research, harnessing the power of the Mecp2 heterozygous knockout mouse model to explore the underlying mechanisms of Rett syndrome and give you the best opportunity to discover your breakthrough.
Our neurodevelopmental disorders areas of expertise: