At PsychoGenics, we recognize the critical nature of reliable preclinical pharmacokinetic and pharmacodynamic services. What sets us apart is our commitment to providing both consistency and quality, all under one roof. This integrated approach simplifies project management, minimizes risks, and reduces time to receive your data.
Comprehensive PK Study Options for Dosing, Sampling and Bioanalysis
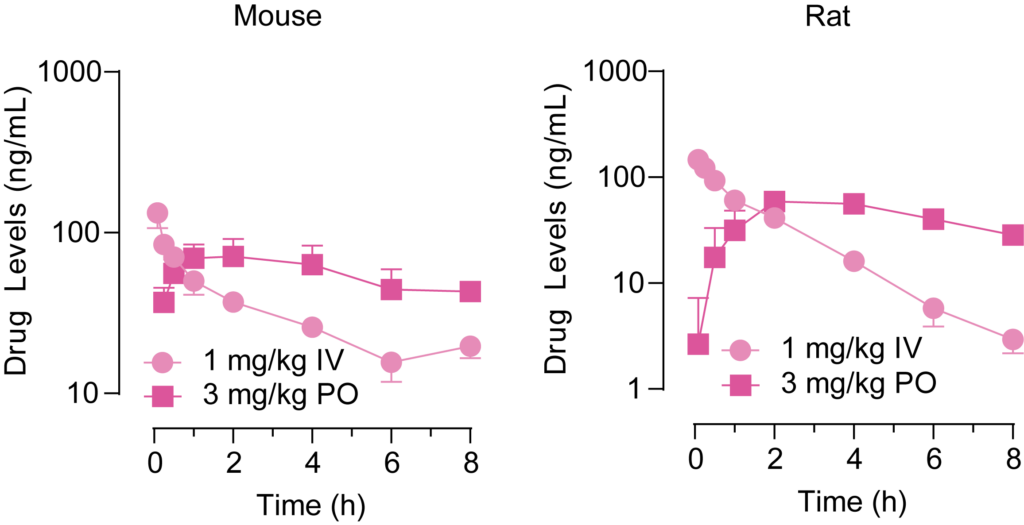
PsychoGenics offers a wide range of options for your rodent in vivo discovery PK studies. In addition to standard dose administration routes such as oral (PO), intravenous (IV), subcutaneous (SC), intraperitoneal (IP), intramuscular (IM), we are skilled at surgery-assisted routes directly into the CNS space such as intracerebroventricular (ICV). We provide an extensive set of sample collection and processing choices for blood, CSF, and tissues, ensuring we cater to the specific sampling requirements of your study.
Our PK sample bioanalysis capabilities for the measurement of exogenous molecules cover your small molecule and protein therapeutic modalities using our ultra performance liquid chromatography (UPLC) with tandem mass spectroscopy (MS/MS), electrochemical detection (ECD), or ELISA based assay systems including MesoScale (MSD) and Luminex.
Combining PD Endpoints
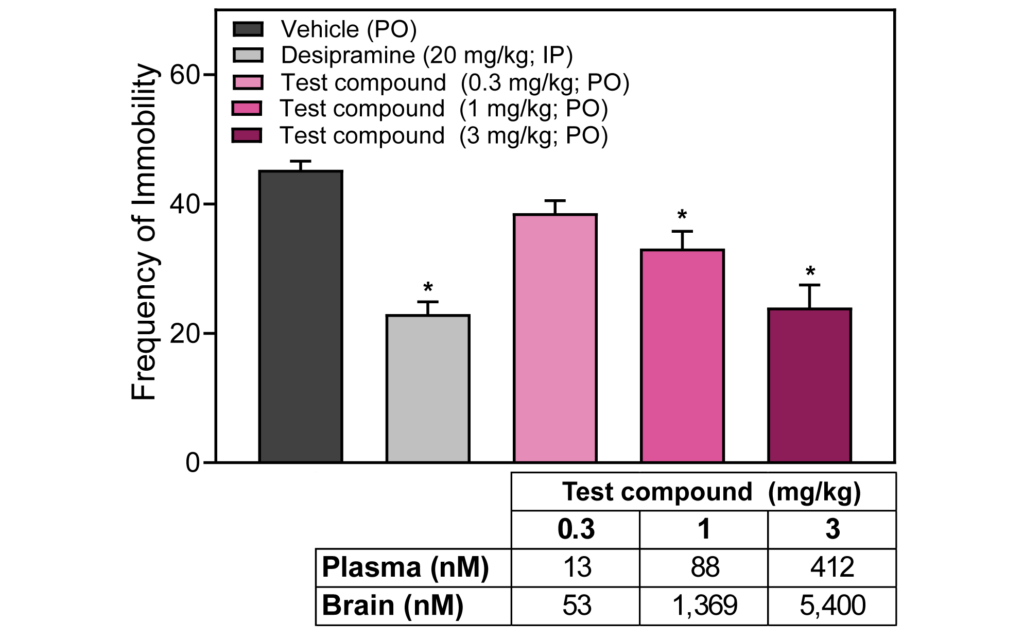
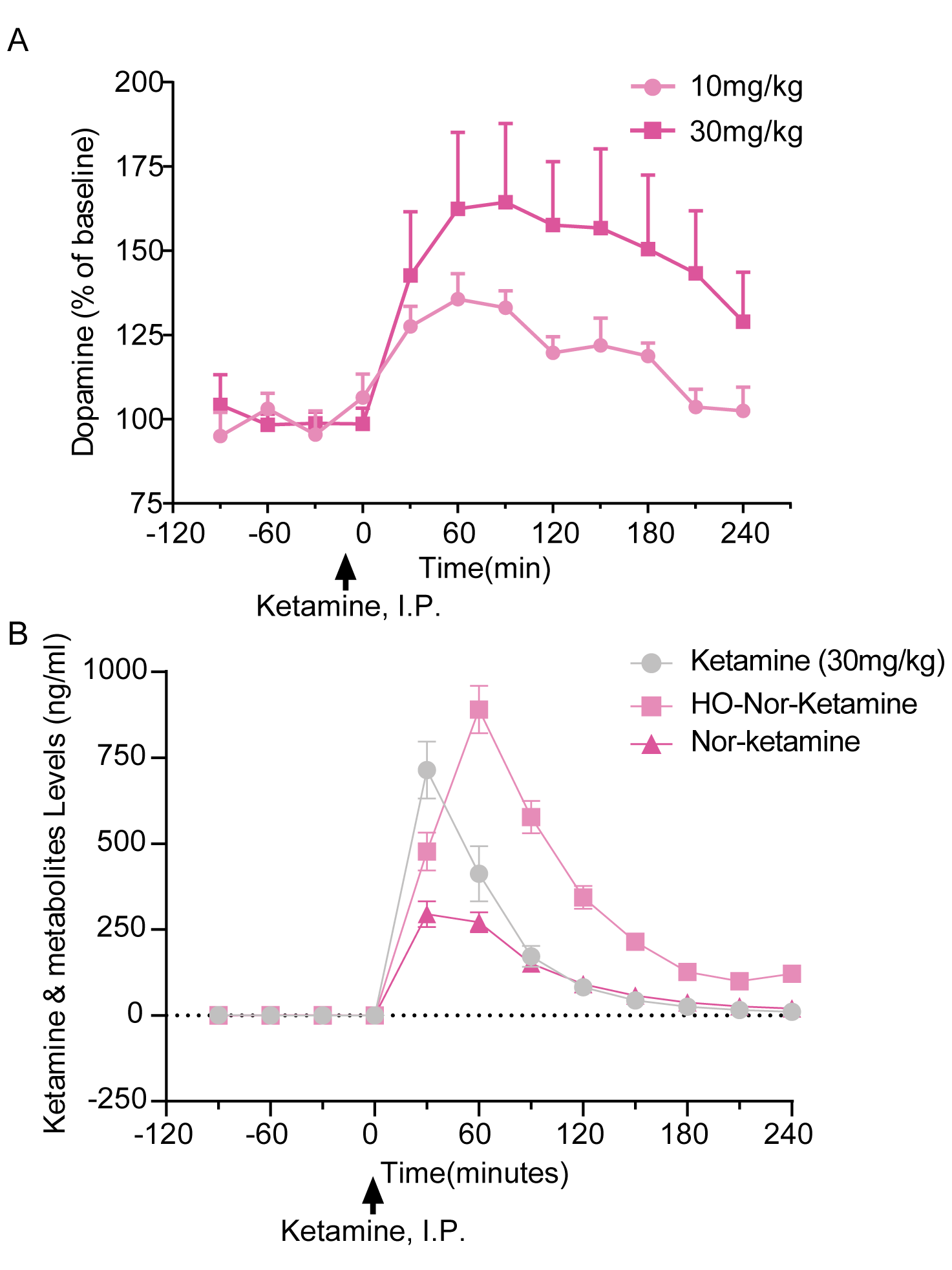
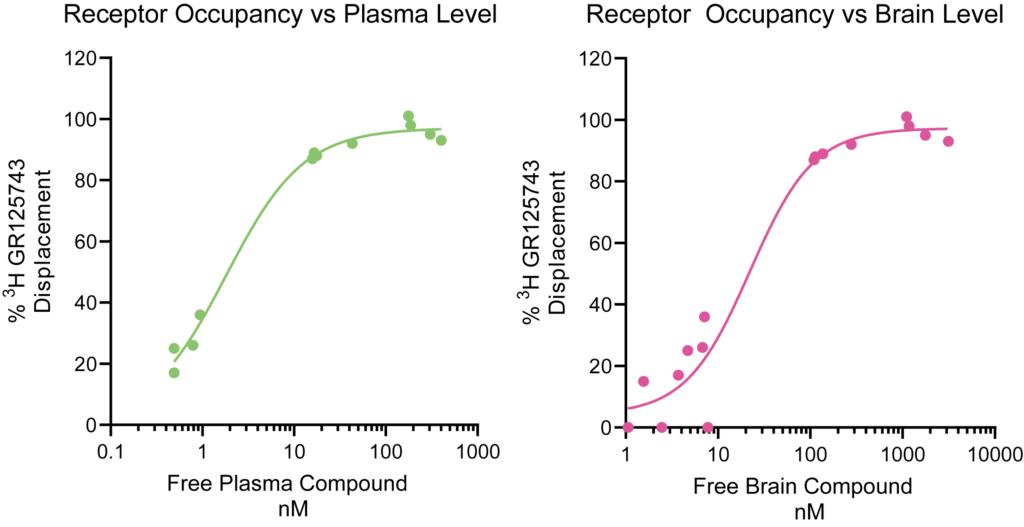
PsychoGenics offers combined preclinical PK and PD studies, enabling a comprehensive understanding of the relationship between drug exposure and its desired therapeutic response, allowing for optimization of dose regimens and prediction of clinical efficacy. PsychoGenics’ full range of capabilities can be deployed to measure PD effects.
- Behavioral and In Vivo Testing
- EEG Studies
- Receptor Occupancy
- Histology and Immunohistochemistry
- Microdialysis
- Ex Vivo Sample Biomarker Analysis
Establishing Pharmacokinetic-Pharmacodynamic (PK/PD) Relationships
PsychoGenics has established methods and assays to measure drug levels from plasma, urine, tissues including brain, spinal cord, and various organs, cerebrospinal fluid (CSF) and extracellular fluid (ECF), the latter by equilibrium in vivo microdialysis. Bioanalysis is conducted using LC-MS/MS for small molecules and peptides (up to 6000 kDa depending on the peptide), or ELISA-based assays such as Meso Scale Discovery (MSD) for measuring larger molecules.